Abstract
Background: Cachexia is a muscle wasting disorder present in 50% of cancer patients, 80% of advanced cancer patients, and is indirectly responsible for 20-30% of cancer-related mortality (von Haehling et al, JCSM. 2016). Prior studies have shown that cachexia is linked to poor treatment response and may be a proxy for an altered immune-state (Coss et al, CCR. 2018). While our pilot work suggested that serum-based cachexia markers are associated with poor survival after chimeric antigen T-cell receptor therapy (CART) (Roy et al., BrJH. 2022), the independent impact of cachexia, defined using consensus criteria, on short- and long-term outcomes after CART has not been explored. Our objective in this study was to evaluate whether consensus weight-based classifications of cachexia have an impact on short- and long-term outcomes after anti-CD19 CART in aggressive B-cell lymphomas.
Methods: Adults with R/R aggressive B-cell NHL treated with anti-CD19 CART between 2015- 2020 from 13 academic centers in the US were identified. Demographic and clinical data were collected and analyzed as univariates via Chi-squared and Kaplan-Meier analysis. Cox multivariable regression analyses assessed impact of significant covariates on survival with α = 0.05. Cachexia (>5% body weight loss (BWL) or 2% BWL with BMI < 20), pre-cachexia (0-5% BWL), and non-cachexia (no BWL) statuses were defined at apheresis using consensus weight-based criteria (Fearon et al., Lancet. 2011).
Results: 433 patients had available weight and BMI data to establish cachexia status. Incidence of cachexia was 37%, pre-cachexia was 18%, and non-cachexia was 43%. Median follow up after CART was 12.4 mo for the entire cohort and 18 mo for survivors. There was no significant univariate association between cachexia and age, sex, performance status, co-morbidity index, CART product, LDH elevation, or IPI score. Significant univariate associations included cachexia patients were less likely to be on a clinical trial (p<0.001), more likely to have auto-transplant pre-CART (p<0.01), and more likely to have ABC cell of origin (p<0.05), while increased lines of pre-CART therapy also trended towards association (p=0.08).
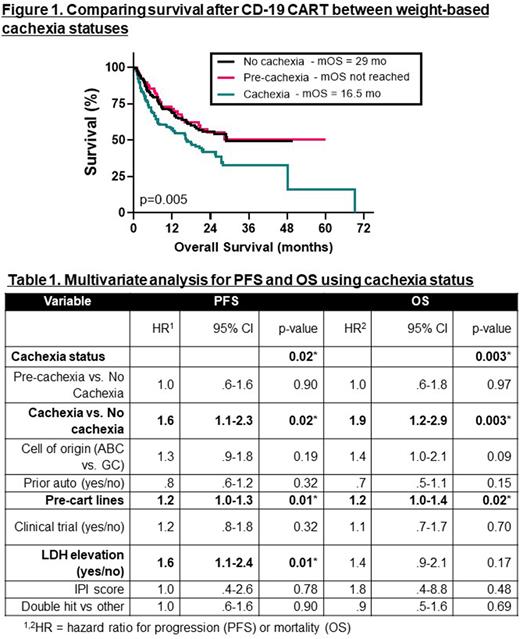
Amongst short-term outcomes, there was no association between cachexia and post-treatment cytokine release syndrome (p=0.93), neurotoxicity (ICANS) (p=0.20), infection (p=0.25), overall response at day 180 (p=0.91), complete response rate at day 180 (p=0.52), or advancement to post-CART salvage therapy (p=0.15). In survival analyses (Figure 1), patients with cachexia had decreased median progression free survival (mPFS) of 6.7 mo, compared with 13 mo for non-cachexia (HR 1.3 (95%CI 1.0 -1.8), p=0.04) and 23.4 mo for pre-cachexia (HR 1.5 (95%CI 1.0-2.2), p=0.03). Additionally, median overall survival (mOS) was 16.5 mo for cachectic patients, significantly lower compared to mOS of 29 mo for non-cachexia (HR 1.7 (95%CI 1.2 - 2.2), p=0.005) and a mOS that was not reached for pre-cachexia.
In a multivariate analysis (Table 1), independent predictors of decreased mPFS were cachexia (HR 1.6 (95%CI 1.1 - 2.3), p = 0.02), number of pre-CART therapies (HR 1.2 (95%CI 1.0 - 1.3), p = 0.01), and elevated LDH at time of CART (HR 1.6 (95%CI 1.1 - 2.4), p = 0.01). Independent predictors of decreased mOS were cachexia (HR 1.9 (95%CI 1.2 - 2.9), p = 0.003), and number of pre-CART therapies (HR 1.2 (95%CI 1.0 - 1.4), p = 0.01).
Conclusion: As durable remission rates remain close to 40%, improved predictors of survival post-CART treatment for lymphoma are needed. In this multi-site study, our findings suggest that cachexia, defined by weight-based criteria, should be included as a prognostic marker for CART. Our data also indicate that, amongst CART patients, the weight/BMI threshold for cachexia criteria (>5% BWL or 2% BWL with BMI < 20) is more prognostically relevant than pre-cachexia (0-5% BWL). While prior mechanistic studies show that cachexia is driven by an altered inflammatory state, our study expands upon this notion. Our data show that although patients with cachexia have similar initial response rates to non-cachectic patients, there may be a long-term dampened immune response in this population that ultimately impacts survival. Further study into cachexia-focused therapies is needed and specific investigation of the role of cachexia in immune evasion mechanisms may yield new targets.
Disclosures
Epperla:Incyte: Speakers Bureau; Novartis: Honoraria; TG Therapeutics: Other: Ad Board; BeiGene: Other: Ad Board; Seattle Genetics: Other: Ad Board; Pharmacyclics: Other: Ad Board. Shouse:Beigene Inc USA: Honoraria; Kite Pharma: Speakers Bureau. Romancik:AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Moyo:Seattle Genetics: Consultancy. Hess:Bristol-Myers Squibb: Consultancy; AstraZeneca: Consultancy, Speakers Bureau; ADC Therapeutics: Consultancy. Moreira:CTI BioPharma: Consultancy; Ingenio Rx: Consultancy. Ma:Bristol Myers Squibb: Consultancy; Janssen: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Research Funding; Juno: Research Funding; Loxo: Research Funding; BeiGene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding. Winter:Daiichi Sankyo: Other: for Spouse, to the University of Chicago, Research Funding; Novartis: Consultancy, Other: for Spouse, to the University of Chicago, Research Funding; Rafael: Other: For Spouse, to University of Chicago, Research Funding; Forty Seven/Gilead: Other: For Spouse, to University of Chicago, Research Funding; Astellas: Other: For Spouse, to University of Chicago, Research Funding; CVS/Caremark: Consultancy, Other: For Spouse; Cellectis: Other: for Spouse, to the University of Chicago, Research Funding; Servier: Consultancy, Other: For Spouse; Merck & Co., Inc.: Honoraria, Research Funding. Pro:Seattle Genetics: Honoraria. Stephens:Newave: Research Funding; TG Therapeutics: Consultancy; Epizyme: Consultancy; AstraZeneca: Consultancy; CSL Behring: Consultancy; Lilly: Consultancy; Genentech: Consultancy; Beigene: Consultancy; AbbVie: Consultancy; Celgene: Consultancy; Novartis: Research Funding; Mingsight: Research Funding; Karyopharm: Research Funding; JUNO: Research Funding; Arqule: Research Funding; Acerta: Research Funding. Danilov:Astra Zeneca: Consultancy, Research Funding; Beigene: Consultancy; Takeda Oncology: Research Funding; Bristol-Meyers-Squibb: Consultancy, Research Funding; GSK: Consultancy; Bayer Oncology: Research Funding; Cyclacel: Research Funding; MEI: Consultancy, Research Funding; Nurix: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Incyte: Consultancy; Morphosys: Consultancy; Genentech: Consultancy; Pharmacyclics: Consultancy. Shah:Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Miltenyi Biotec: Consultancy, Research Funding; Kite Pharma: Consultancy; TG therapeutics: Consultancy; Bristol Myers Squibb: Consultancy; Novartis: Consultancy; Lilly Oncology: Consultancy, Honoraria; Epizyme: Consultancy. Cohen:Janssen: Consultancy; Lilly Oncology/Eli Lilly: Consultancy, Research Funding; BMS/Celgene: Research Funding; Astrazeneca: Consultancy, Research Funding; HutchMed: Consultancy, Research Funding; Novartis: Research Funding; Takeda: Research Funding; Kite Pharma/Gilead: Consultancy; Genentech: Research Funding; Aptitude Health: Consultancy; BeiGene: Consultancy, Research Funding. Barta:Kyowa Kirin: Consultancy, Honoraria; Acrotech: Honoraria; Daiichi Sankyo: Consultancy; Janssen: Other: Independent Data Monitoring Committee member; Seagen: Honoraria; Affimed: Consultancy. Torka:Targeted Oncology, Physician Education Review: Honoraria; Epizyme: Consultancy; Lilly USA: Consultancy; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy; Genentech: Consultancy. Gordon:Zylem: Current equity holder in private company, Current equity holder in publicly-traded company, Patents & Royalties: Patent on nanoparticles for lymphoma therapy; BMS: Research Funding; Ono Pharmaceuticals: Consultancy; Janssen: Other: DSMB. Karmali:Pharmacyclics: Consultancy, Other: Advisory Board; BMS/Celgene: Consultancy, Research Funding; Genentech/Roche: Consultancy, Other: Advisory Board; Calithera: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; AstraZeneca: Other: Advisory Board, Speakers Bureau; Karyopharm: Consultancy; Kite: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; Morphosys/Incyte: Consultancy, Other: Advisory Board, Speakers Bureau; Takeda: Research Funding; Eusa: Consultancy; BeiGene: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal